With a new rollout of updated 2023 vaccines, we’re updating this piece. We’ll bring you that guidance here soon!

What is Coronavirus (COVID-19)?
Coronavirus (COVID-19), pronounced “kr-ow-nuh-vai-ruhs” is a highly contagious respiratory illness. Symptoms are usually mild and similar to a cold or the flu. They typically appear 2-14 days after exposure to the virus. Initially, symptoms of COVID-19 only included cough, shortness of breath, and fever. However, it’s likely that you have COVID-19 if you have at least 2 of the following symptoms: fever, chills, repeated shaking with chills, muscle pain, sore throat, and/or new loss of taste or smell. It’s important to remember people with respiratory diseases such as asthma or cystic fibrosis, those with a weakened immune system, or people over the age of 60 years old may experience severe symptoms that could be life-threatening.
How do people get this virus?
The virus is found in an infected person’s mucous, saliva and/or sputum (secretions from the lungs). COVID-19 spreads when an infected person sneezes or coughs close to a non-infected person. The virus can also be spread by touching contaminated objects or surfaces then touching your face.
Who is at risk for getting COVID-19?
Unfortunately, anyone who lives, works, or goes to school in the community is at risk for contracting COVID-19. The virus can affect anyone and everyone–no matter your color, race, age, gender, or sexual orientation, coronavirus has the ability to infect anyone. Older people are at higher risk of complications, but anyone can get really sick from COVID-19. According to John Hopkins University and Medicine, hundreds of thousands of people living in the United States have been diagnosed with COVID-19, since the first confirmed case in December of 2019.
Are there different types of coronavirus vaccines available?
Yes, right now there are three different types of coronavirus vaccines available in the United States. However, it is very likely that more vaccines will be developed and available around the world as time moves forward.
| Vaccine Name | Number of shots | Approved for: | Overall Effectiveness | % Effective in Preventing Serious Illness (may not need hospitalization) | % Effective in Preventing Hospitalization |
| Pfizer-BioNTech (Comirnaty*)
(Those aged 5-11 years receive a smaller dose |
Two-dose series (meaning two separate shots), given at least 21 days after the first dose. | Anyone over the age of 16 years old. (though there is emergency FDA approval for use in those 5-15 years old). | 95% effective in preventing COVID-19 after both doses have been administered in people between over the age of 16 years old. | 92% | 87% |
| Moderna | Two-dose series (meaning two separate shots), given at least 28 days after the first dose. | Anyone over the age of 12 years old. | 94.1% effective in preventing COVID-19 after both doses have been administered in individuals over the age of 12 years old. | 100% | 89% |
| Janssen/ Johnson & Johnson Vaccine* | One-dose series | Anyone over the age of 18 years old. | 66.3% effective in preventing COVID-19 with one shot. | 85% | 100% |
*Pfizer has received full approval by the FDA. This means it can be marketed by the brand name of Comirnaty.
It’s very important to mention that if you receive either the Pfizer-BioNTech or the Moderna vaccine for your first dose, then you must receive the same one for your second dose. In order to ensure virus immunity (protection from the virus), you must receive the second dose at least 21 or 28 days after the first dose (see table above) and it must be from the same manufacturer (either Pfizer or Moderna) as your first dose. All three of these vaccines are extremely effective. Current recommendations are to get any of the 3 vaccines when they become available to you.
What are the side effects?
Everyone is different, but it’s not uncommon for younger people between the ages of 12 to 55 years old to experience mild to moderate side effects following the COVID vaccine. It’s thought that those under 12 years will experience similar side effects. All three vaccines share the same side effects, here are some common ones:
Injection Site (Arm):
- Redness
- Swelling
- Pain
- Rash, a.k.a. “COVID Arm” (red, itchy rash that can appear days after injection)
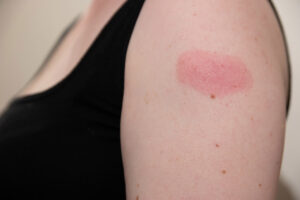
A great way to help ease any pain or discomfort at the sites is by applying a cool, clean, cloth to the injection site. Also make sure you move and use your arms a little more after the injection, as this helps move the injected vaccine around.
Rest of the Body:
- Headache
- Fever
- Chills
- Muscle Aches
- Tiredness
- Nausea
Make sure you drink lots and lots of fluids and wear loose fitting clothing. These tips will help if you develop a fever after your vaccine!
Fortunately, none of these side effects are harmful, and they should resolve in 1-2 days following the vaccine. If you experience side effects after vaccine one (Remember: Pfizer and Moderna are a two-part series), you must get vaccine number two to build immunity. Talk to your health care provider (HCP) about taking pain and fever relieving medications after your vaccine. It is very important that you do not take any fever or pain relieving medication before your vaccine, as it can decrease the effectiveness of the vaccine.
What happen with the Janseen (Johnson & Johnson) Vaccine?
On April 13th, 2021, the Food and Drug Administration (FDA) ordered a pause on the administration of the Janseen (Johnson & Johnson) vaccine. The pause was put in place after several women in the United States (who were under the age of 50 years old) reported blood clots, a rare but serious side effect. After further research, the FDA confirmed that the vaccine was safe for administration and the pause was lifted in late April. If you have questions about receiving the Janseen (Johnson & Johnson) vaccine, we recommend that you speak with your health care provider (HCP) before receiving the vaccine.
What are the long-term effects? How safe is it?
The CDC reports that over three hundred and fifty million million people living in the United States have been vaccinated against COVID-19. All three vaccines in the US have been tested through clinical trials and all three were considered safe and effective by the Food and Drug Administration (FDA).
The vaccine only has emergency use authorization (EUA), what does that mean?
There are only 2 major differences between FDA approval and EUA FDA approval. The first difference is that with EUA FDA approval, vaccine testing and vaccine production occur at the same time, this allows for quicker distribution once the vaccine is completely approved. The second difference has to do with which type of licensing is granted. When vaccine developers submit for FDA approval they submit a “Biologic License Application” (BLA). Once the application is reviewed (meaning they look at the same number of clinical trials and testing as a EUA vaccine) and the vaccine is deemed safe, they are granted FDA approval. The same exact process has to occur when vaccine developers submit for a EUA approval it’s just done at a faster rate.
What if I have allergies?
Everyone who receives the new COVID-19 vaccine, must be monitored for a minimum of 15 minutes after receiving the new vaccine, regardless of whether or not you have allergies.
However, if you have ever had an allergic reaction that has caused you to experience shortness of breathe, difficulty breathing, or anaphylaxis, you will be monitored for a minimum of 30 minutes. It’s important to discuss your allergies with your health care provider (HCP) before your vaccine appointment. Your HCP can help determine which vaccine is safest for you, depending on your allergies.
What if I am allergic to Polyethylene glycol 3350 (PEG 3350)?
Polyethylene glycol 3350 (PEG 3350) is the main ingredient found in a common laxative called MiraLAX. This ingredient is also found in both the Pfizer Bio-NTech and Moderna vaccine, but not in the Janssen Johnson & Johnson vaccine. If you are allergic to PEG 3350, it’s very important that you speak with your health care provider, before you schedule your COVID-19 vaccine appointment.
How is the shot given?
All three vaccines are an intramuscular (IM) injection, meaning it’s injected into a muscle, just like the flu shot! Most people will receive the injection into their deltoid muscle, located in the upper to middle part of the arm.
Does it matter which vaccine I receive?
No. All three vaccines are equally as good and reliable. Some people may have allergies to certain components, however. Be sure to talk to your HCP if you have a history of severe allergies.
How are the Pfizer-BioNTech and Moderna vaccines made?
Both the Pfizer-BioNTech and Moderna vaccines are made with mRNA technology. This means that the mRNA is a blueprint for the cell on how to make a piece of the spike protein unique to SARS-CoV-2 (the virus that causes COVID-19). Since only a piece of the protein is made, it does not do any harm to the person who is vaccinated. The cell then creates the protein and displays it on the cell surface – where the immune system goes to work creating antibodies and activating T-cells to fight off what it thinks is an infection. This is also why some people may feel sick after receiving the vaccine. It’s your body learning how to fight and should resolve in a day or two. If you continue to feel unwell, or have any questions, reach out to your HCP.
How is the Janssen/Johnson & Johnson vaccine made?
The Janssen/Johnson & Johnson vaccine is a viral vector vaccine which means it uses a modified version of a different harmless virus to deliver instructions on how to make a piece of the spike protein unique to SARS-CoV-2. The harmless virus (called a vector) will enter a cell in our body and use it to produce the spike protein. Similar to the Pfizer-BioNTech and Moderna vaccines, the cell creates the spike protein and displays it on the surface, where our immune systems recognizes that it doesn’t belong there and starts creating antibiodies and activation T-cells. Again, this is why some people feel uncomfortable after receiving the vaccine as their body learns to fight the virus. The difference with this vaccine is really just how the cell receives the blueprint.
OK, so I got my COVID vaccines. Am I immune now?
In order to achieve full protection from the COVID vaccines, you must wait at least two weeks from the last injection. For example: if you received the Pfizer or Moderna vaccine, than you won’t receive full protection until two weeks after your LAST injection. However, if you received the Janssen Johnson & Johnson vaccine, you will receive full protection two weeks after the initial injection, because it’s only one injection! It’s very important that even though you’ve received full immunity from the vaccine, that you continue to wear your mask, wash your hands, and maintain social distance. As above, these vaccines are not 100% effective at preventing illness, so these prevention is still important. Also, the majority of people have yet to be vaccinated and we are not at herd immunity yet, so continuing to take these prevention measures are our best shot at stopping COVID.
Should I get a booster?
Since the vaccines are new, we are learning something new every day. This does not mean that the vaccines are not safe. However, we are learning that the vaccine protection tends to decrease after six months. Boosters are now becoming available for people who would to receive them due to being at higher risk. If you are wondering if you are eligible for a booster, talk to your HCP.
Our health guides are developed through a systematic, rigorous process to ensure accuracy, reliability, and trustworthiness. Written and reviewed by experienced healthcare clinicians from Boston Children's Hospital, a Harvard Medical School teaching hospital and consistently ranked as a top hospital by Newsweek and U.S. News & World Report, these guides combine clinical expertise, specialized knowledge, and evidence-based medicine. We also incorporate research and best practices from authoritative sources such as the CDC, NIH, PubMed, top medical journals, and UpToDate.com. Clinical specialists and subject matter experts review and edit each guide, reinforcing our commitment to high-quality, factual, scientifically accurate health information for young people.

